
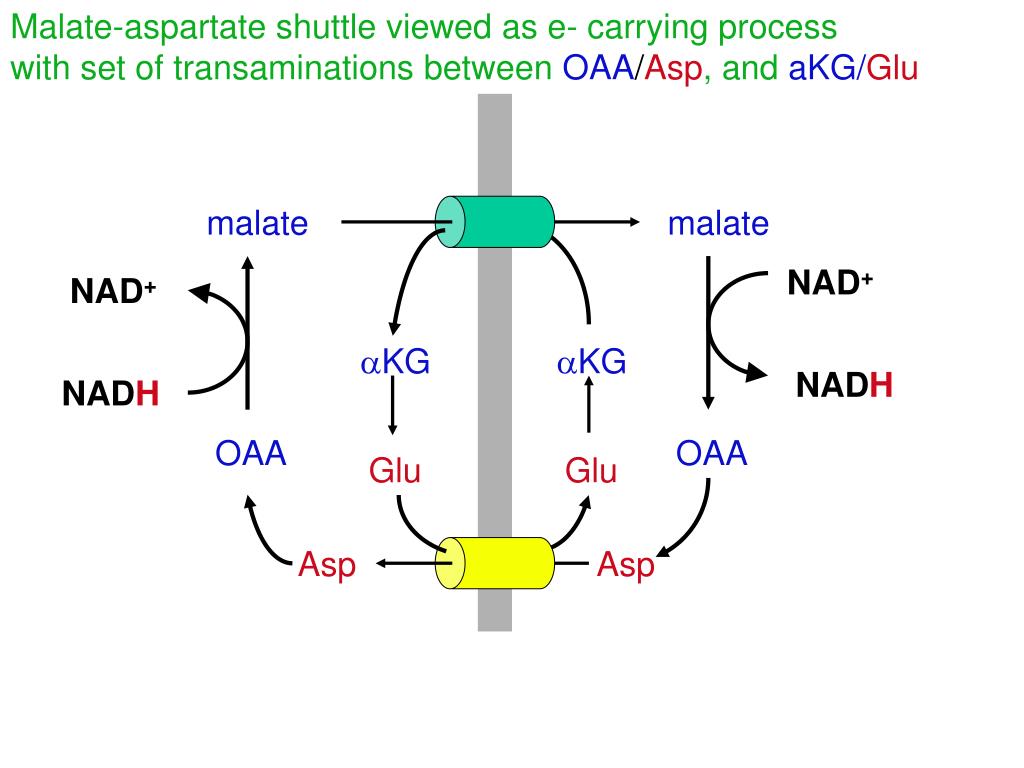
Schematic Diagram Showing the Essential Role of the Malate-Aspartate NAD(H) Redox Shuttle in the Re-oxidation of Cytosolic NADH 4 Defects in the MAS have been described due to mutations in genes encoding mitochondrial malate dehydrogenase ( MDH2 ) and both aspartate-glutamate carriers ( SLC25A12, SLC25A13 ). 3 The MAS provides a mechanism for net transfer of NADH reducing equivalents across the inner mitochondrial membrane. 3 Since the inner mitochondrial membrane is relatively impermeable to NAD + and NADH, 4 NAD(H)-redox shuttles exist. 1, 2 NADH produced in cytosolic NAD-linked dehydrogenase reactions, mainly during glycolysis, is re-oxidized to NAD + inside the mitochondria. These enzymes are part of the malate-aspartate shuttle (MAS), a key player in intracellular NAD(H) redox homeostasis ( Figure 1). Both isoforms catalyze the reversible interconversion of oxaloacetate and glutamate into aspartate and α-ketoglutarate. This is a pyridoxal 5′-phosphate (PLP)-dependent enzyme that exists as cytosolic (GOT1) and intramitochondrial (GOT2) isoforms.

Here we describe four affected individuals with a metabolic encephalopathy with epilepsy due to a defect in the mitochondrial isoform of glutamate oxaloacetate transaminase or aspartate aminotransferase (GOT EC 2.6.1.1). Although inborn errors of metabolism do not represent the most common cause of these encephalopathies, their early identification is of utmost importance, since many require targeted therapeutic measures beyond that of common antiepileptic drugs, either to control seizures or to decrease the chance of neurodegeneration. Identification of an underlying diagnosis is paramount for personalized management. Infantile-onset encephalopathies with epilepsy often are devastating disorders with major consequences for the life of affected individuals and their families. Our data provide a mechanistic basis for the biochemical abnormalities in GOT2 deficiency that may also hold for other MAS defects. The two treated individuals reacted favorably to their treatment. Both pyridoxine and serine synergistically rescued embryonic developmental defects in zebrafish got2a morphants. Knockdown of got2a in zebrafish resulted in a brain developmental defect associated with seizure-like electroencephalography spikes, which could be rescued by supplying pyridoxine in embryo water. Correcting the highly oxidized cytosolic NAD-redox state by pyruvate supplementation restored serine biosynthesis in GOT2-deficient cells. De novo serine biosynthesis was impaired in fibroblasts with GOT2 mutations and GOT2-knockout HEK293 cells. GOT2, a member of the malate-aspartate shuttle, plays an essential role in the intracellular NAD(H) redox balance. GOT2 enzyme activity was deficient in fibroblasts with bi-allelic mutations. GOT2 encodes the mitochondrial glutamate oxaloacetate transaminase. Zebrafish and mouse models were used to validate brain developmental and functional defects and to test therapeutic strategies. Functional consequences of observed mutations were tested by measuring enzyme activity and by cell and animal models. The epilepsy was serine and pyridoxine responsive. In-depth metabolic studies in individual 1 showed low plasma serine, hypercitrullinemia, hyperlactatemia, and hyperammonemia. Whole-exome sequencing was used to investigate the disease etiology in four children from independent families with intellectual disability and epilepsy, revealing bi-allelic GOT2 mutations. Early-infantile encephalopathies with epilepsy are devastating conditions mandating an accurate diagnosis to guide proper management.


 0 kommentar(er)
0 kommentar(er)
